Applications
Dr
Matt Greenway (currently at McMaster University, Canada) and Dr Orla Hardiman
(currently at Trinity College Dublin) have studied ALS-specific mutations in
angiogenin. Nature Genetics February 2006.
Prof
Jochen Prehn, of the RCSI, and his collaborators have carried out key studies
building on these findings showing that angiogenin is expressed preferentially
in the spinal cord and is neuroprotective to motorneurons, both in cultures and
in a mouse model of ALS (Greenway et al., Nat Genetics, 2006; Kieran et al., J
Neurosci, 2008.). Angiogenin delivery has led to a functional improvement and
increased life span when applied after disease onset in this mouse model.
Currently both protein delivery-based and viral delivery-based therapeutic
approaches are being developed by the team.
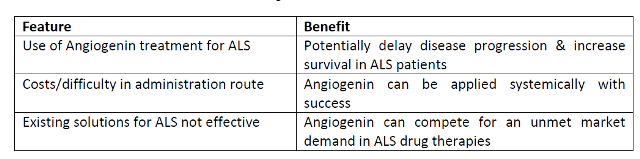
Advantages
This
opportunity points to the development of new therapeutic products for ALS, an
incurable and invariably fatal disease where only one disease-modifying therapy
currently exists. Given that ALS is a relatively rare disease there is the
potential that new agents will be awarded “orphan drug” status.
-
The new approach is based on breakthrough work on a gene not previously
associated with ALS
-
There is the potential that disease-modifying drugs in ALS may have therapeutic
effects in other more common neurodegenerative diseases
A patent has been issued under
the title “Treatment of ALS and variants thereof consisting of primary lateral
sclerosis (PLS) or spinal muscular atrophy (SMA)” (US patent number 7,659,243),
relating to the use of angiogenin in the treatment and prevention of ALS and
other neurodegenerative diseases. Additional intellectual property applications
are currently under prosecution.
Contacts:
Dr.
Gearóid Tuohy, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2,
Ireland.
Email: gearoidtuohy@rcsi.ie Tel: +353 1 4022362
Dr
Aoife Gallagher, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2,
Ireland.
Email: aoifegallagher1@rcsi.ie. Tel: +353 1
4022394
Dr
Liz Moran, Enterprise Ireland, East Point Business Park, Dublin 3.
Email: liz.moran@enterprise-ireland.com. Tel: +353 1
7272696
Principal Investigator:
Prof. Jochen Prehn, Dept. of
Physiology & Medical Physics, Royal College of Surgeons, 123 St Stephen’s
Green, Dublin 2, Ireland. Email: JPrehn@rcsi.ie Tel: +353 1 4022255