Introduction
Bone grafts are used clinically in the treatment of many forms of
bone tissue defect (e.g. fracture alignment/non-union, critical-sized defects,
maxillo-facial surgery and spinal fusion). They actively promote healing and new
bone formation (osteogenesis) using a biocompatible, osteoconductive and
osteoinductive graft structure that provides mechanical support and promotes
osteogenesis. Historically, the gold standard has been either autografts
(patient’s own tissue) or allografts (donor tissue).
Whilst autografts are still widely used, drawbacks such as donor
site morbidity are shifting the market rapidly toward the use of allografts and
synthetic bone graft substitutes. However, due to the inherent drawbacks of
these materials, development of an ideal bone graft substitute made from
materials already found within the body is paramount.
Bone grafts are second only to blood transfusions on the list of
transplanted materials worldwide. The total U.S. bone graft and bone
graft substitutes market revenues were $ 1.3 billion in 2006. This is expected
to reach $ 3.3 billion in 2013 with the compound annual growth rate (CAGR) from
2006 to 2013 expected to be 13.8%. The total European bone graft substitute
market alone is expected to reach $120m by 2010.
Technology
The RCSI bone graft substitute, HydroxyColl, combines the two main
constituents of bone tissue, namely hydroxyapatite and type I collagen, in the
form of a three dimensional construct that possesses the requisite intrinsic
mechanical strength, architecture and biocompatibility for successful use as a
commercial bone graft substitute, improving on currently available products.
The development of HydroxyColl has culminated in a number of
successful pre-clinical studies, highlighting the excellent regenerative
potential of this biodegradable bone graft substitute (Fig. 1).
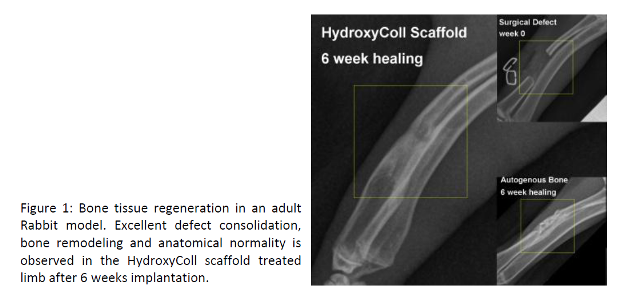
The RCSI team has developed a novel proprietary
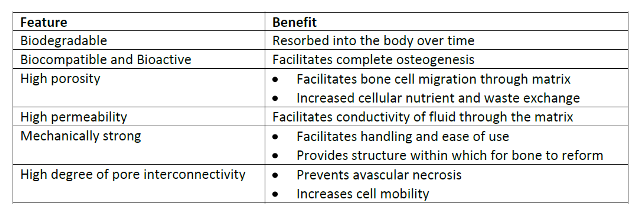
collagen/hydroxyapatite (CHA) based technology that has the high levels of
permeability, porosity, and pore interconnectivity required to ensure scaffold
viability and promote osteogenesis in vivo while offering variable mean
pore size for cell-specific biological activity. In summary, this technology
offers a 3-D scaffold with the prerequisite mechanical, biocompatible and
bioactive characteristics for successful use in vivo.
Applications
A global ageing population coupled with increasing prevalence of
obesity and sedentary lifestyles calls for increased innovation and advancements
in implant design. As much as 60% of all injury-related physician visits are
linked to the musculoskeletal system. New technologies are currently in
development that could drastically change the nature of orthopaedic surgery
creating key opportunities for synthetic, manufactured products.
There is an unmet need for a successful bioactive, load bearing,
bone substitute material that promotes osteogenesis in vivo. This is
something of a ‘holy grail’ in the medical device industry.
Advantages
This novel CHA based technology will offer a real alternative not
only to existing commercially available synthetic products but also to auto and
allografts.
A significant increase in the scaffold compressive strength,
as well as its permeability (an important determinant of in vivo
viability) has been achieved with no detrimental effect to either the
scaffold porosity or biocompatibility.
HydroxyColl
is a biomimetic and biodegradable alternative to autogenous bone and a viable
and cost effective bone regeneration aid for clinical
use.
Contacts:
Dr Aoife Gallagher, RCSI Technology Transfer, 123 St Stephen’s
Green, Dublin 2, Ireland.
Email: aoifegallagher1@rcsi.ie. Tel: +353 1 4022394
Dr Liz Moran, Enterprise Ireland, East Point Business Park, Dublin
3. Email: liz.moran@enterprise-ireland.com.
Principle Investigator:
Prof. Fergal J
O’Brien & Dr. John Gleeson, Dept. of Anatomy, Royal College of Surgeons, 123
St Stephen’s Green, Dublin 2, Ireland. Email: fjobrien@rcsi.ie or
johngleeson@rcsi.ie